Chemical reactions are the bedrock of chemistry, where substances interact and transform into different entities, often resulting in the formation of new compounds. Among the fascinating elements that influence these reactions, catalysts play a pivotal role. A catalyst, unlike reactants, is not consumed in the reaction but rather accelerates the process. Understanding the mechanisms through which catalysts enhance reaction rates is crucial for various fields, including industrial chemistry, environmental science, and biochemistry.
To bode well for a comprehensive understanding, one must first delve into the fundamentals of chemical reactions. Generally, chemical interactions occur as bonds between atoms break and form anew, leading to the creation of different molecules. Factors such as temperature, concentration, and pressure can significantly affect the velocity of these reactions. However, the introduction of a catalyst can alter the reaction landscape entirely.
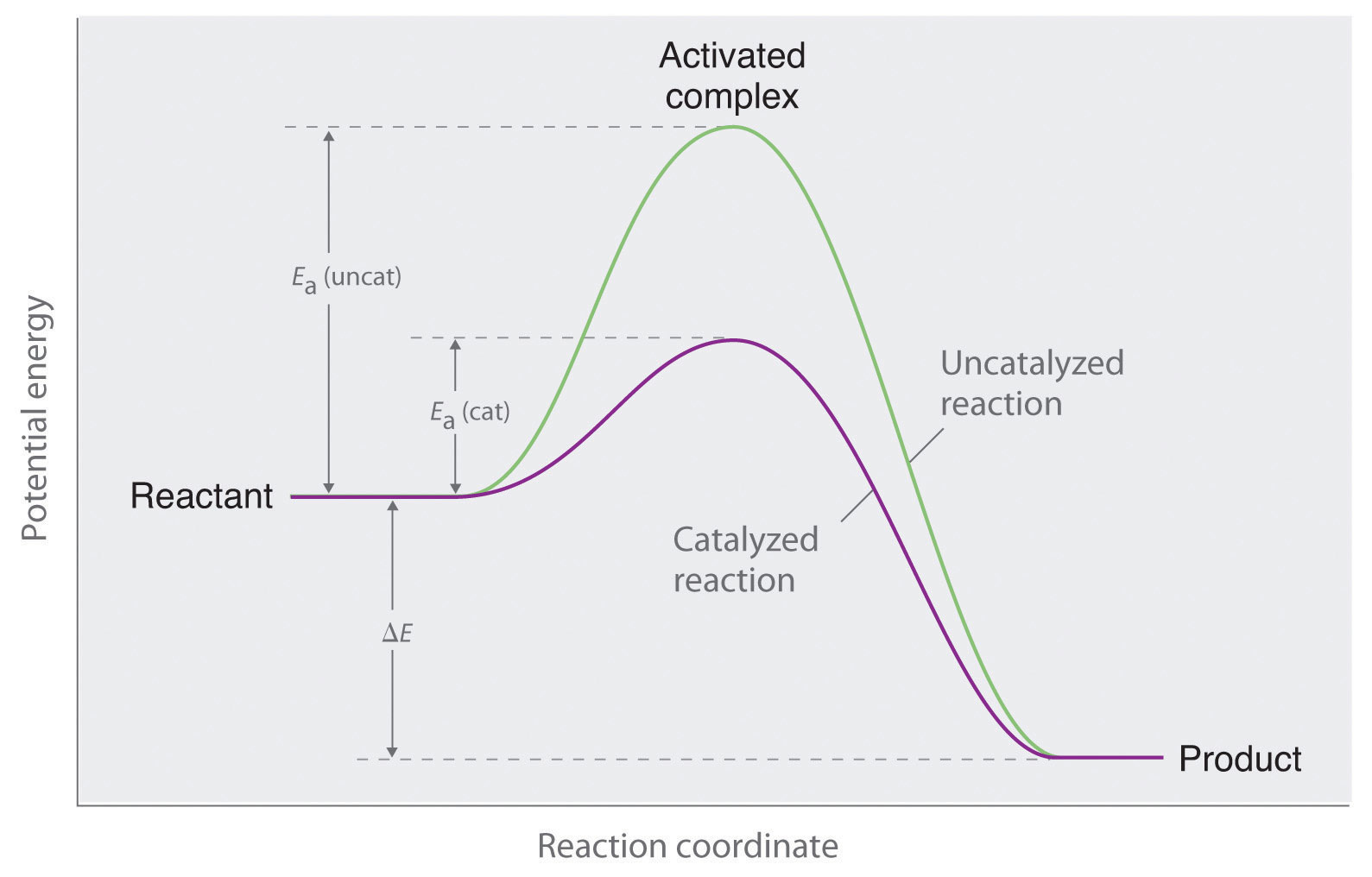
To understand how a catalyst works its magic, consider the concept of activation energy—the minimum energy required for a reaction to occur. In any chemical reaction, reactants must overcome this activation energy barrier. Catalysts lower this barrier, providing a more favorable energy profile for the reaction to proceed. By allowing reactions to occur with less energy input, catalysts make it possible for a reaction to proceed at a faster rate, enabling industries to operate more efficiently and with reduced energy costs.
There are two primary categories of catalysts: heterogeneous and homogeneous. Heterogeneous catalysts exist in a different phase than the reactants, often in solid form, while the reactants are typically in a gas or liquid phase. A classic example of a heterogeneous catalyst is platinum used in catalytic converters in automobiles. In contrast, a homogeneous catalyst exists in the same phase as the reactants and often participates in the same solution. An example is the use of acids or bases in the esterification process, which occurs in a liquid phase.
The mechanism by which catalysts accelerate reactions can be intricate. Enzymes, the biological catalysts, exhibit extraordinary specificity and efficiency. These protein-based molecules not only lower activation energies but often stabilize transition states, making it easier for reactions to proceed. The enzyme-substrate complex is a testament to the intricate dance of molecular interactions, where substrates align precisely on the enzyme’s active site, allowing for optimal reaction conditions.
Transition states are crucial in understanding catalysis. A transition state represents the highest energy configuration that reactants achieve before transforming into products. Catalysts provide an alternative pathway with a lower transition state energy, which ultimately results in a faster reaction. By stabilizing this transient configuration, catalysts facilitate the conversion of reactants to products with remarkable efficiency.
Beyond traditional chemical reactions, catalysts are also instrumental in various environmental processes. For instance, the use of catalysts in exhaust systems helps convert harmful gases like carbon monoxide into less harmful substances, significantly reducing air pollution. This application underscores the role of catalysts in making industrial processes more environmentally friendly, aligning with the principles of sustainable chemistry.
The application of catalysts extends to the realm of renewable energy. Catalysts are crucial in processes such as electrolysis, where water is split into hydrogen and oxygen gas. This hydrogen can then be harnessed as a clean energy source, opening gateways to a more sustainable energy future. The development of new, efficient catalysts is an ongoing area of research, focusing on the discovery of materials that can perform optimally under varying environmental conditions.
However, not all catalysts are created equal. Catalyst poisoning is a term that denotes the deterioration of catalytic activity due to the deactivation of catalyst sites. Poisoning can occur if contaminants bind to the active sites, preventing the proper interaction with reactants. The management of catalyst stability and efficiency is vital in industrial settings to ensure optimal performance and longevity.
Moreover, researchers are increasingly considering the recyclability of catalysts, which contributes to the circular economy. The ability to efficiently recycle and reuse catalysts is becoming paramount as industries aim to minimize waste and reduce their ecological footprint. Innovations in catalyst design and optimal usage conditions are continually evolving, promising advancements in green chemistry.
In summary, catalysts are invaluable entities in the chemistry realm that offer profound impacts on the rates of chemical reactions. By lowering activation energies and enabling alternative reaction pathways, they foster efficiency in various chemical processes. From industrial applications to environmental management and renewable energy solutions, the role of catalysts is multifaceted and pivotal. With ongoing advancements in research and applications, the future of catalysis holds exciting prospects that extend beyond the laboratory to tangible benefits in our everyday lives. As the world moves towards sustainable practices, the spotlight on catalysts will only intensify, challenging scientists to innovate and reimagine our chemical interactions.