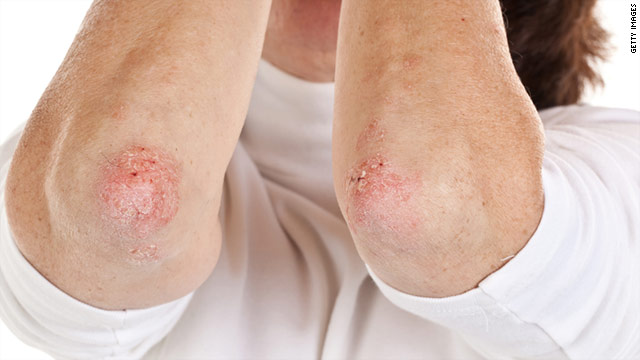
The landscape of dermatological treatment has undergone significant transformation in recent years. Among the most intriguing advancements is the advent of biologic medications intended to treat psoriasis, a chronic autoimmune condition characterized by red, scaly patches on the skin. Recognized for their efficacy, these treatments have garnered considerable attention. However, recent announcements by the FDA regarding potential risks associated with certain psoriasis drugs have stirred discussions within both the medical community and among patients.
Historically, the therapeutic approach to psoriasis has been multifaceted, encompassing topical therapies, phototherapy, and systemic medications. Biologics, which target specific components of the immune system, have emerged as a novel and powerful means of managing severe cases. Yet, as the FDA emphasizes the scrutiny of these drugs, the conversation surrounding their safety and efficacy gains a critical edge.
The FDA’s recent guidance sheds light on specific cardiovascular risks linked to some psoriasis medications. This has raised eyebrows and prompted a deeper inspection into the drug development and approval process. While the initial studies suggested no significant cardiac risks, new data has emerged, necessitating a reevaluation of the benefits versus the potential hazards involved. This dichotomy of healing and risk is not unique to psoriasis medications; rather, it persists across many pharmaceutical innovations.
One cannot overlook the psychological ramifications for patients reliant on these treatments. The fear of exacerbation of symptoms juxtaposed against the potential for serious side effects creates an emotional battleground for those affected. For many, the prospect of severe psoriasis may compel individuals to prioritize immediate symptomatic relief over long-term safety considerations. This complexity deepens the fascination surrounding psoriasis medications and highlights the often-unexamined dilemmas inherent in modern medical practices.
Moreover, the implications extend beyond individual patient experiences. The larger healthcare system grapples with the necessity of balancing innovative drug approval and patient safety. Regulatory bodies like the FDA play a pivotal role in this equilibrium, tasked with ensuring that the medications provided are both safe and effective. Their meticulous evaluations serve as a reminder that the pharmacological landscape is not merely a realm where efficacy reigns supreme but a nuanced arena requiring careful deliberation.
Ultimately, as more patients learn about the FDA’s findings, the discourse surrounding psoriasis medications will likely amplify. Patients, providers, and researchers alike must navigate this labyrinthine situation, weighing the benefits of breakthrough therapies against the backdrop of possible risks. Through continued dialogue and research, advancements in dermatological care can be both innovative and grounded in patient safety, fostering a healthcare environment where efficacy and safety coexist harmoniously.