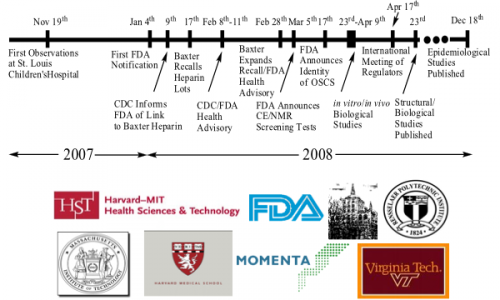
The Heparin contamination crisis of 2008 stands as a sobering reminder of the intricate web woven by global supply chains. This troubling episode, which originated in China, reveals not only the vulnerabilities of pharmaceutical processes but also elicits a profound reassessment of safety standards within the industry. It is a narrative whereby the implications of oversight and negligence stretch far beyond borders, enveloping patients in a veil of uncertainty.
Heparin, an anticoagulant derived from animal tissues, was widely used during surgeries and for patients with cardiovascular afflictions. In the grip of this crisis, adverse events culminated swiftly, revealing that contaminated batches led to severe allergic reactions and, tragically, fatalities. Initial investigations unearthed that the pathogen was not a mere byproduct of manufacturing but a malign strain introduced during the synthesis process of heparin. This revelation sent ripples through the healthcare community, sparking outrage and concern.
The spotlight shone brightly on the facilities in China that produced the active pharmaceutical ingredient (API) for heparin. Questions arose regarding regulatory efficacy and due diligence. How could such a destructive contaminant infiltrate a medication so widely used? The answer lay in a perilous amalgam of regulatory laxity and the relentless quest for cost-cutting measures. This quest culminated in the use of substandard raw materials, particularly a counterfeit ingredient known as oversulfated chondroitin sulfate, which bore a striking resemblance to heparin but harbored devastating consequences.
The scandal uncovered an unsettling truth: the globalization of pharmaceutical manufacturing had outpaced the governance required to ensure safety and quality. Regulatory bodies, such as the Food and Drug Administration (FDA), were compelled to reevaluate their oversight mechanisms, recognizing the limitations of inspection frequencies and the efficacy of existing frameworks. The crisis urged stakeholders to explore a paradigm shift toward more robust, integrated global regulations to fortify supply chain integrity.
In the aftermath, healthcare providers and consumers alike were left grappling with an uncomfortable paradigm. Trust, once a cornerstone of the patient-physician relationship, was shaken. The public, now more than ever, demanded transparency and accountability from pharmaceutical companies, compelling many to introspect on the intricate balance of cost and safety. This incident serves as a prophetic warning—that vigilance is paramount, for the stakes are undeniably high.
Ultimately, as the industry commenced its post-crisis introspection, the necessity for a collective commitment to safety became indubitable. Enhanced scrutiny of supply chains, coupled with the bolstering of regulatory frameworks, promises not only to safeguard humanity’s health but also to restore faith in an industry characterized by innovation. Hence, the chapter that began with contamination could very well evolve into one of renewed vigor and unwavering dedication to patient safety.